
AstraZeneca, the European Medicines Agency and the World Health Organization have all maintained that there is no direct link between the vaccine and reported blood clots. /Johan Nilsson/TT News Agency/AFP
AstraZeneca, the European Medicines Agency and the World Health Organization have all maintained that there is no direct link between the vaccine and reported blood clots. /Johan Nilsson/TT News Agency/AFP
The European Medicines Agency's (EMA) executive director, Emer Cooke, on Tuesday said that more detailed scientific evaluation is needed before a conclusion is made on the safety of the AstraZeneca COVID-19 vaccine and its possible link to blood clots in vaccinated people, but that the EMA is "firmly convinced" of the vaccine's benefits.
"At present, there is no indication that vaccination has caused these conditions. They have not come up in the clinical trials and they are not listed as known or expected side events with this vaccine," Cooke told the press conference.
Clinical trials had shown "very small numbers of blood clot developments," she added.
The Amsterdam-based EMA's expert safety committee was meeting on Tuesday to assess new information and investigate each individual potentially adverse event and would reach a conclusion on "whether there are any further actions that need to be taken" at a special meeting on Thursday, Cooke said, without specifying what the measures could be.
"We need to have the facts first. We cannot come to a conclusion until we've done a thorough scientific analysis," Cooke explained.
However, Cooke added that the EMA remains "fairly convinced that the benefits of the AstraZeneca vaccine in preventing COVID-19 … outweigh the risk of these side effects," but there is a "serious concern" that requires detailed scientific consideration.
"A situation like this is not unexpected when you vaccinate millions of people. It's inevitable that you have rare or serious instances of illnesses that occur after vaccination," Cooke explained.
00:22
The EMA director also addressed the theory that individual batches of the AstraZeneca vaccine were suspected of being behind the adverse effects – after Austria and others banned a batch – saying the EMA did initially investigate this, but that as more events are reported "more batches are involved and therefore it is unlikely it is a batch specific event."
On the different approach taken by EU states, Cooke said those are being taken in the context of information available at the national level, adding: "It is the country's prerogative to do so."
"It is our responsibility to focus on the science associated with these risks and whether there is scientific evidence to show if they are causally related to the vaccine," Cooke explained.
Asked about similar reports from the Pfizer and Moderna vaccines regarding blood clotting in those who had taken the shot, Cooke said the EMA is looking into adverse events associated with all vaccines, but that the current focus was on the AstraZeneca jab.
"It looks like there are similar numbers coming in from across the world," she said.
02:02

World Health Organization experts are also meeting on Tuesday to discuss the vaccine.
The EMA approved the AstraZeneca vaccine for people of all ages on January 29, but its roll-out was troubled from the start, with several countries initially saying it should not be used on older people.
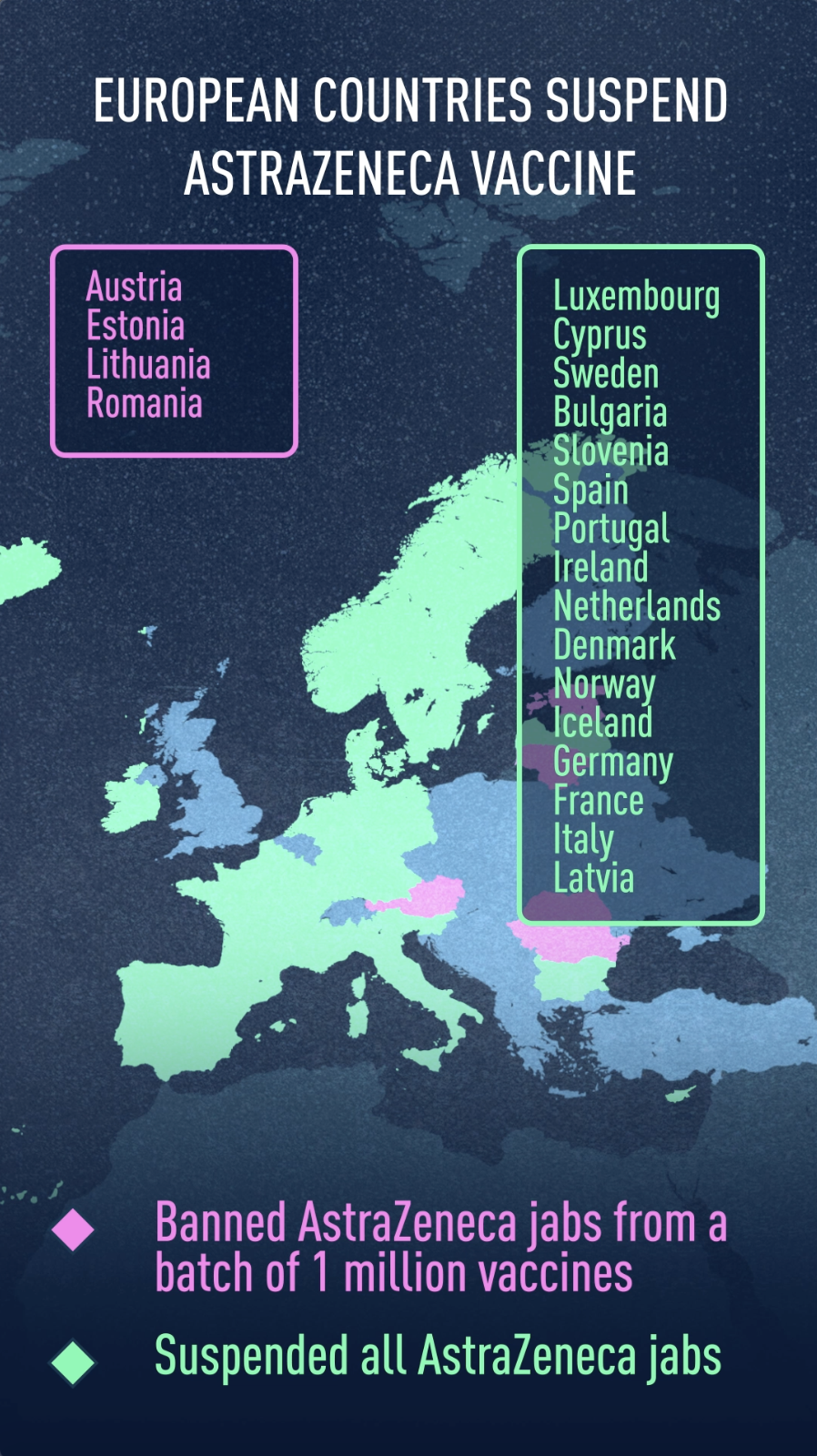
About 15 countries, mostly in Europe, have halted the use of AstraZeneca's COVID-19 vaccine over fears of the safety of the jab following several cases of blood clots or brain hemorrhages in people after receiving the inoculation, with some deaths reported.
Sweden, Luxembourg and Cyprus on Tuesday became the latest nations to halt the roll-out, following moves by Germany, Italy, France, Spain, Denmark, Norway, Iceland, the Netherlands, Portugal, Slovenia, Ireland, Bulgaria, Latvia, Romania and Iceland.
Earlier in March, Austria, Estonia and Lithuania suspended their use of a batch of AstraZeneca shots (ABV5300) after Austria said it was investigating a death from coagulation disorders and an illness from a pulmonary embolism. For its part, Romania has suspended another batch, ABV2856, due to fears related to the formation of blood clots.
But AstraZeneca and medical experts in Britain have said there is no evidence of clots being caused by the jab or that they are occurring in greater numbers or frequency than in the general population.
AstraZeneca, which developed the vaccine with the University of Oxford, has said a review of its safety data revealed no evidence of an increased risk of blood clots. The review covered more than 17 million people vaccinated in the UK and the EU.
The World Health Organization and the European Medicines Agency have also insisted the shot is safe and that there is no link between it and reported blood clots.
In a statement on Monday, the EMA said that "many thousands of people develop blood clots annually in the EU for different reasons" and that the number of incidents in people who have been vaccinated "seems not to be higher than that seen in the general population."
UK Prime Minister Boris Johnson on Tuesday defended the safety of the AstraZeneca vaccine, writing in The Times newspaper: "That vaccine is safe and works extremely well."
He added: "It is being made in multiple places from India to the U.S., as well as Britain, and it is being used around the world."
Video animation: Steve Chappell and Ben Wildi

