02:26
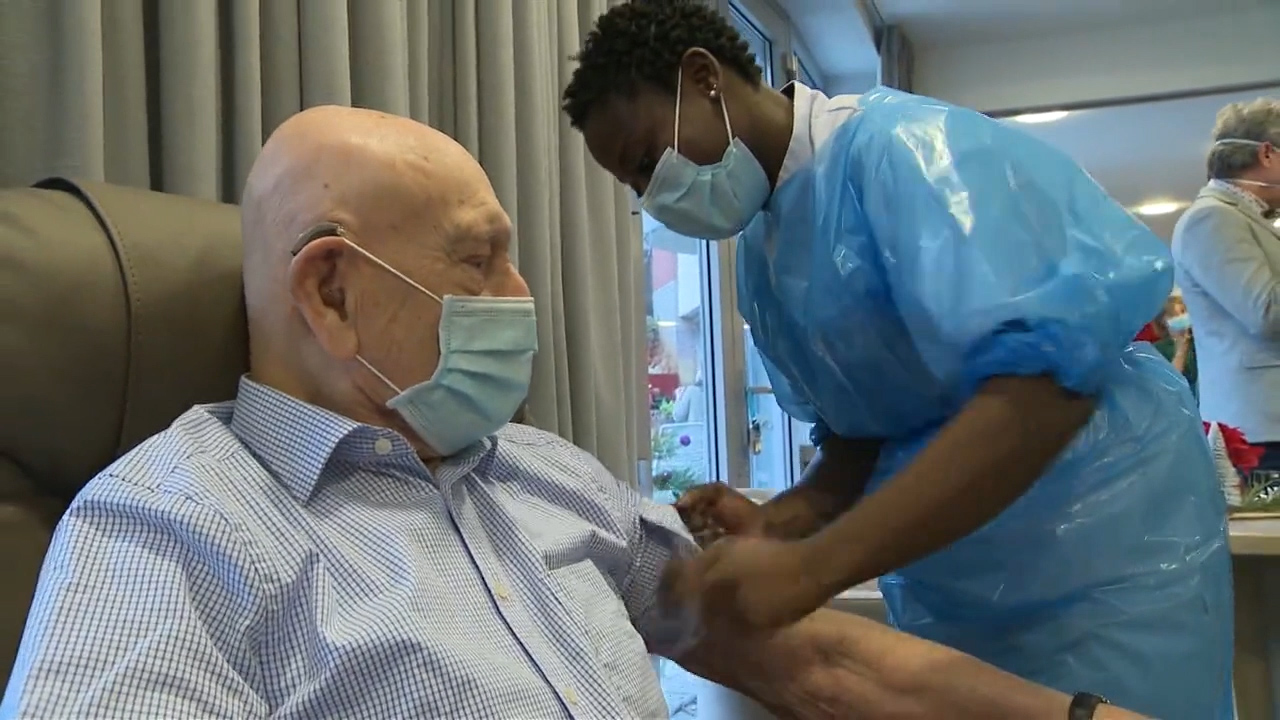
After a pilot week between Christmas and New Year's Day, Belgium's coronavirus vaccination campaign officially launched on Tuesday and first in line were 200,000 care home residents and staff.
But as the immunization drive picks up steam, critics are questioning the pace of the roll-out after Vaccine Day morphed into Vaccine-Week-And-A-Half.
The first ceremonial jabs were administered on December 28, right after the Pfizer-BioNTech vaccine received approval from EU health regulators and the European Commission.
But as of Sunday, only 700 people had received their first dose.

Critics said the roll-out was far too glacial, especially when compared with the speed of delivery in the UK, Israel, and the U.S., where millions have already rolled up their sleeves.
Officials admitted Belgium lagged its peers, but claimed it was for good reason.
"We need to learn first and this is what we did in December," said Steven Van Gucht, a virologist and interfederal COVID-19 spokesperson.
"We've heard some examples abroad, in Germany where they gave five times higher doses. Sometimes there were problems with the storage of the vaccine, so they had to throw away vaccines."
With details of a redrawn plan expected on Friday, the Coronavirus Task Force is now revising its immunization strategy and the federal health minister has said no vaccines should be left frozen, adding that "they all have to be used as soon as possible."
Deliveries from Pfizer, which is producing the vaccine at its plant in Puurs, Belgium, have also been delayed.
The company promised that 600,000 doses would be delivered in January, but that was slashed in half due to supply line issues.

Belgium health ministry spokesperson Yves Van Laethem said a logistical issue in the second half of December prevented the delivery of the Pfizer vaccines. /Pfizer
Belgium health ministry spokesperson Yves Van Laethem said a logistical issue in the second half of December prevented the delivery of the Pfizer vaccines. /Pfizer
Brussels fights back
The European Commission, which spearheaded the bloc's vaccine procurement, also came under fire. In an interview with the German newspaper Bild am Sonntag, the leader of Germany's Christian Social Union, Markus Soder, said Brussels failed to purchase enough of the Pfizer-BioNTech shot and had been slow in the roll-out.
"Obviously, the European purchasing procedure was inadequate," he told the paper. "It is difficult to explain that a very good vaccine is developed in Germany but is vaccinated more quickly elsewhere.”
The Commission defended itself and pointed to six signed contracts with vaccine developers, giving them access to 2 billion doses. That, once secured, is up to each member state to purchase and distribute the shots.
"I don't think that the issue is really the number of vaccines, it is the fact that we are at the beginning of a roll-out," said Commission chief spokesperson Eric Mamer.
"We're all judging this as if this campaign is over. In fact, the campaign is just starting."
European health regulators also added to the roll-out pace as the European Medicines Agency (EMA) has only approved the Pfizer-BioNTech shot and did so weeks after regulators in the UK and the U.S. gave it the nod.
It's also weeks behind on the Moderna vaccine, which is expected to be approved on Wednesday.
The EMA has defended its slower approach, which uses the same standards of quality, safety, and efficacy as any other EU medicine.

