
Countries and organizations have called on pharmaceutical companies to share their COVID-19 vaccine technology to help meet global supply. /Bruna Prado/File/AP
Countries and organizations have called on pharmaceutical companies to share their COVID-19 vaccine technology to help meet global supply. /Bruna Prado/File/AP
Almost four months since developers of several COVID-19 vaccines announced positive results in large-scale trials and some received emergency approval for use in lightning-fast time, mass-vaccination programs are in full swing in many parts of the world.
But with fears mounting that current vaccine drives are concentrated in richer nations, several organizations and countries are calling for vaccine producers to license their technology and know-how in order to boost production and supply in less-developed countries.
To date, more than 475.6 million vaccine doses have been administered worldwide, equal to 6.1 per 100 people. In other terms, 3.6 percent of the world's population has received at least one dose of a COVID-19 jab, according to data compiled from government sources by the University of Oxford's Our World in Data project.
While this may be a very impressive achievement in such a small amount of time, the data also show there is already a stark difference in where the vaccines have been administered, with many countries (mostly in the Global South) yet to report a single inoculation.
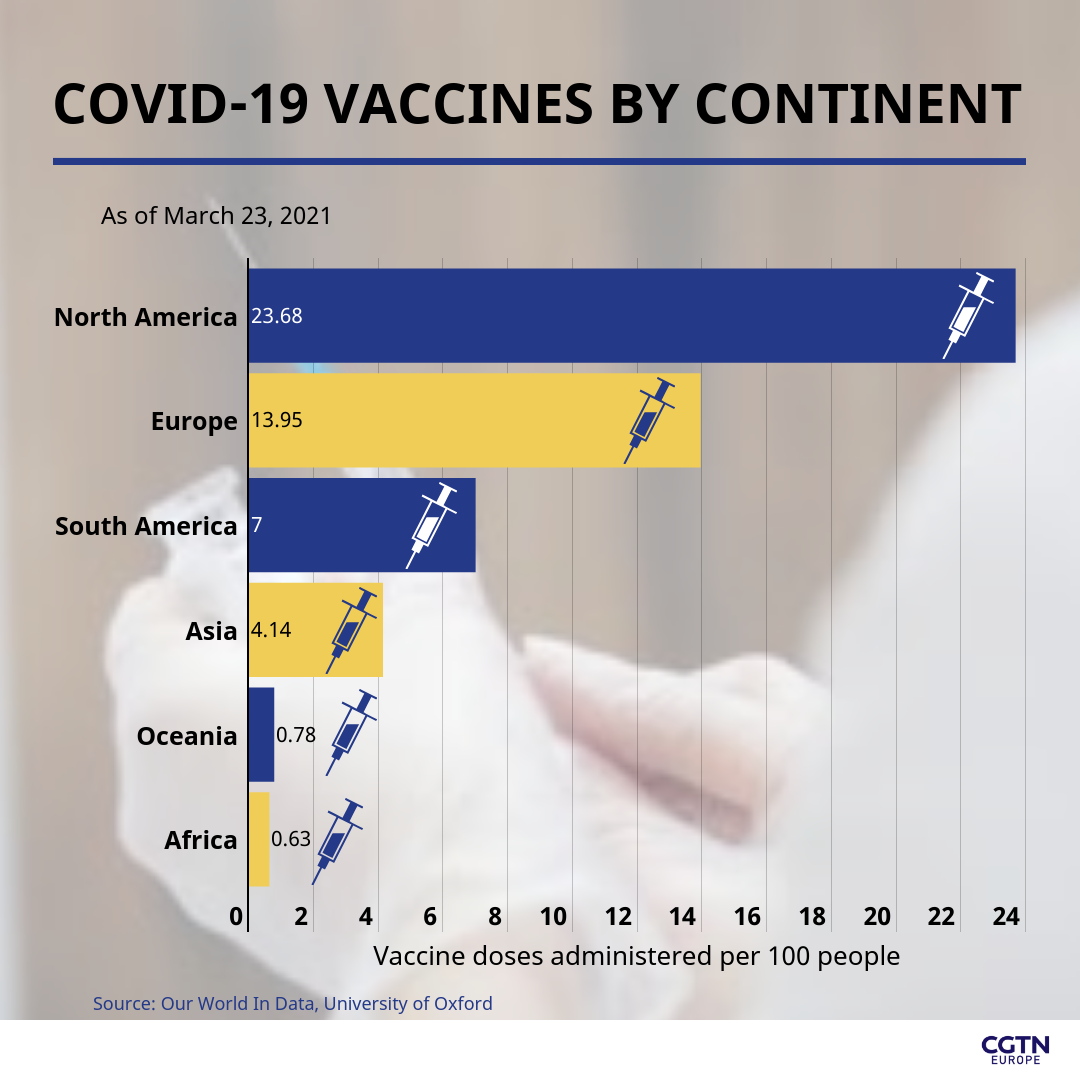
The difference can clearly be seen between continents, as North America and Europe have to date administered 23.68 and 13.95 vaccines per 100 people respectively, compared with Africa, which has administered 0.63 vaccines per 100 people. The Asian continent stands at 4.14 jabs administered per 100 people, while South America has administered 7.0 per 100 people so far.
With the pace of production of the shots (which are mainly taking place in Europe and the U.S.) not getting any faster due to manufacturing delays, as well as shortages of raw materials, countries that are unable to afford to reserve vaccines are left at a growing disadvantage. According to some estimates, most of Africa and parts of Asia and South America are not expected to achieve widespread vaccination until 2023.
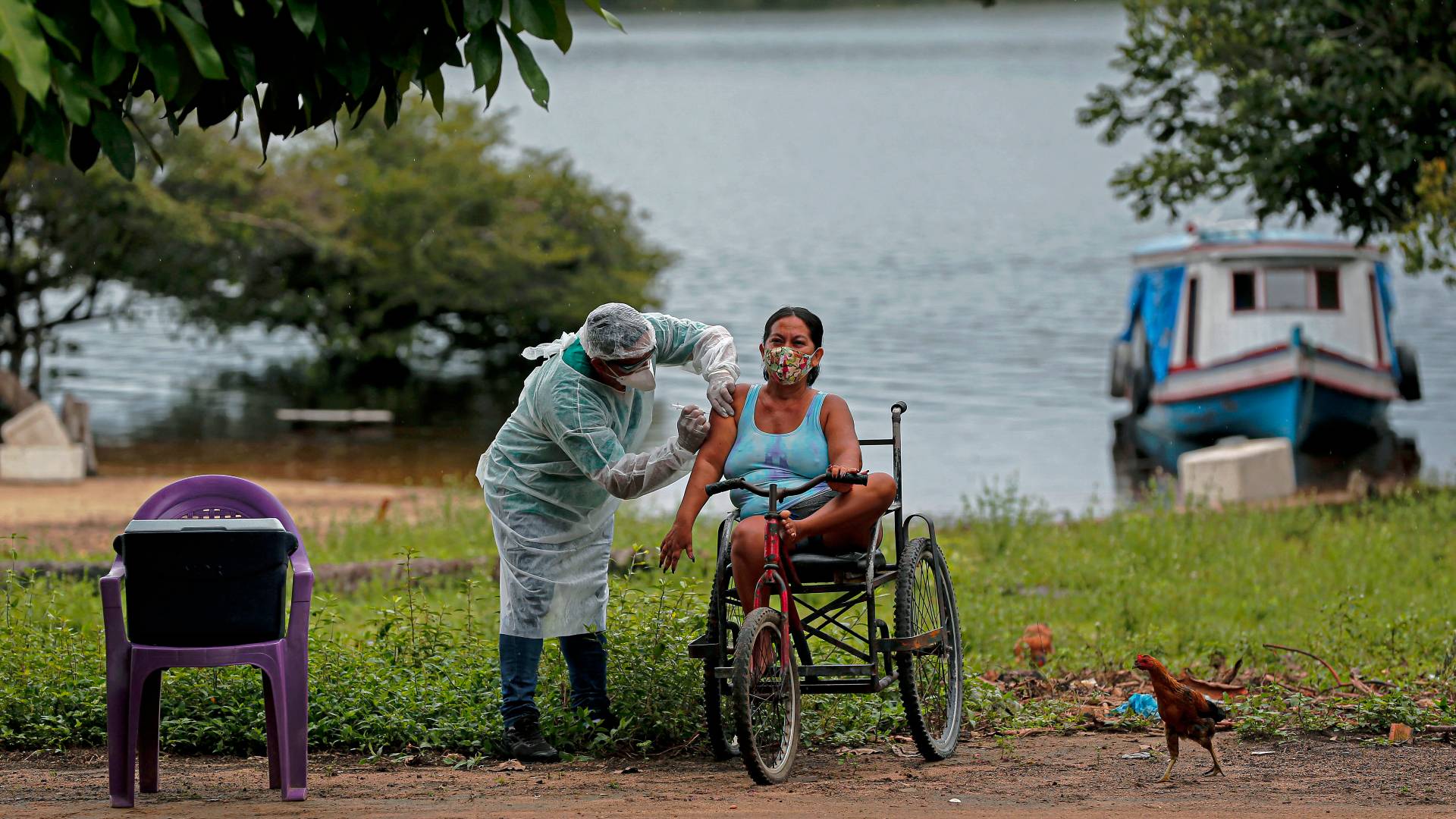
The WHO and others have raised fears about COVID-19 vaccine inequality. /Tarso Sarraf/AFP
The WHO and others have raised fears about COVID-19 vaccine inequality. /Tarso Sarraf/AFP
Sharing intellectual property
The World Health Organization (WHO) addressed the issue, with its Director-General Tedros Adhanom Ghebreyesus describing the continuing vaccine inequity as "grotesque," and "a moral outrage."
WHO emergencies chief Mike Ryan also warned: "We've missed a huge opportunity to bring vaccines onboard as a comprehensive measure. It is not only a catastrophic moral failure, but it is an epidemiologic failure and it is a failure in public health practice."
The solution, according to the WHO and others, is for COVID-19 vaccine producers to license their technology to other manufacturers in order to boost supplies and distribute them much faster and easier.
Many public health experts agree and have publically called on companies to do more to ensure vaccine supplies are not hoarded.
"Pharmaceutical companies could license their vaccines on a non-exclusive basis, either permanently or for a limited period of time, so that other manufacturers with production capacity could produce the vaccines to meet the tremendous global demand," Kayvan Bozorgmehr, a medical doctor and professor in public health at Bielefeld University in Germany, told CGTN Europe.
But with no companies, as of yet, agreeing to do so, several countries have pushed for another solution. This solution, being discussed at the World Trade Organization, supported by more than 100 countries and the African Union, is for a temporary waiver of intellectual property (IP) rights for the new COVID-19 vaccines.
However, the initiative is facing strong opposition by the EU and U.S. governments in particular, with drug companies in the U.S. lobbying the Biden administration to block the request.
This has led many to offer an alternative, more forceful approach, in the form of governments granting permission to someone else to produce a patented product, or compulsory licensing.
"If companies do not voluntarily give up their intellectual property (IP) for the vaccines, governments could issue compulsory licenses, a mechanism that can be activated through regulations of the World Trade Organization while compensating the IP holder," Bozorgmehr explained.
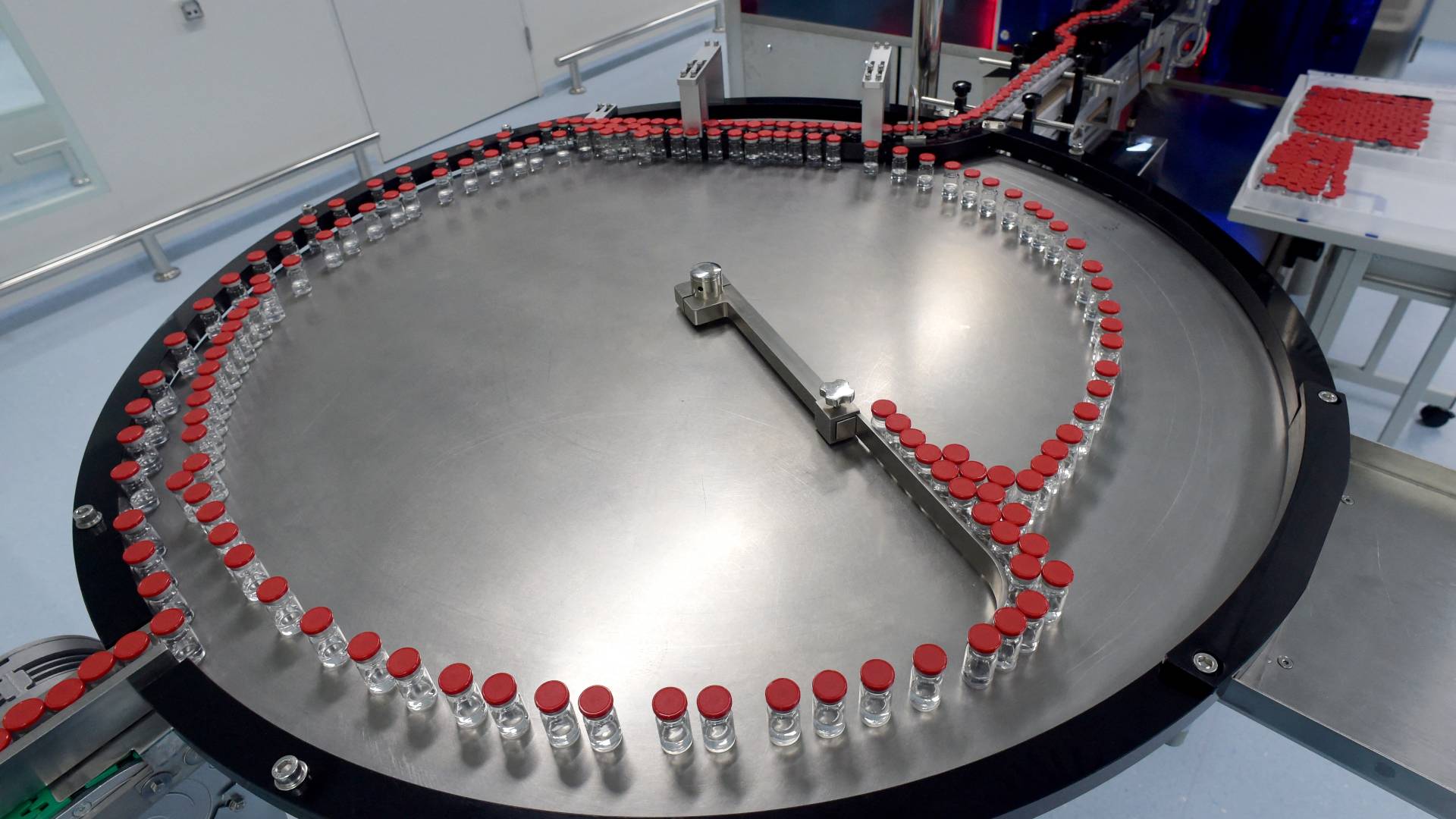
Vaccine manufacturers have so far refused to join the WHO's COVID-19 Technology Access Pool for open access to licensing. /Olga Maltseva/AFP
Vaccine manufacturers have so far refused to join the WHO's COVID-19 Technology Access Pool for open access to licensing. /Olga Maltseva/AFP
Benefits of equitable access to COVID-19 vaccines
The case for vaccine makers to share their licensing and IP openly, has also been made by drug-producing factories around the world, the owners of which say they could start producing hundreds of millions of COVID-19 vaccines on short notice if only they had the blueprints and technical know-how.
According to the Associated Press, one such factory is the Incepta plant in Bangladesh. Its owner, Abdul Muktadir, said it is currently operating at just a quarter of its capacity and is ready and willing to help with COVID-19 vaccine production.
Bozorgmehr explained that open licensing "would allow pharmaceutical companies and biotech industries, also in low- and middle-income countries, to produce vaccines based on the existing know-how. This would increase, decentralize and diversify vaccine production to better satisfy the tremendous global demand and the resulting competition would bring down prices, leading to more equitable access."
He added: "At the moment, other producers can only produce vaccines if they enter bilateral agreements with the IP holders, and very few have done so."
Some COVID-19 vaccine producers have made exclusive licensing deals outside Europe. One of these is AstraZeneca, which has been praised by the WHO and lauded as an example for others to follow.
On Monday, Ghebreyesus said AstraZeneca is "the only company that has committed to not profiting from its COVID-19 vaccine during the pandemic" and the only vaccine developer that has made a significant contribution to vaccine equity, "by licensing its technology to several other companies." This includes the Republic of Korea's SKBioScience and the Serum Institute of India, which are producing more than 90 percent of COVAX-distributed vaccines.
A year ago, the WHO, along with Costa Rica, launched a mechanism called the COVID-19 Technology Access Pool, or C-TAP, designed precisely to promote an "open-science model," where licensing would occur "in a non-exclusive, transparent manner, to leverage as much manufacturing capacity as possible." So far, no manufacturer has agreed to participate in the pool. In fact, Pfizer's CEO Albert Bourla, last year called the concept "nonsense," and even "dangerous."

South Africa joined other countries to push, through the WTO, for a temporary waiver of intellectual property (IP) rights for the new COVID-19 vaccines. /Themba Hadebe/AP
South Africa joined other countries to push, through the WTO, for a temporary waiver of intellectual property (IP) rights for the new COVID-19 vaccines. /Themba Hadebe/AP
Drawbacks for manufacturers
Thomas Cueni, director general of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) rejected the WHO's criticism and calls for free licensing, saying it showed "a lack of understanding for the complexity of vaccine manufacturing and the global supply chain."
He added in a statement that the scaling up of vaccine production was on track thanks to partnerships among vaccine makers in the developing world and developed nations, citing deals between AstraZeneca and Novavax with the Serum Institute in India, and between Johnson & Johnson with Aspen Pharma in South Africa and Biologic E in India.
For vaccine makers that are opposed to free licensing, they argue doing so would hamper innovation, would not help overcome shortages due to the complex process of manufacturing the COVID-19 vaccine, and they need to cover costs for the development of vaccines.
But Bozorgmehr and other public health experts and organizations have said these arguments "are not valid." "First, there is no evidence that free knowledge hampers innovation. Second, while shortages will not be overcome immediately, publicly accessible know-how would allow any manufacturer to rearrange their production ... this would create competition and help overcome monopolies," Bozorgmehr explained.
As for the argument on costs, the long-held model in the pharmaceutical industry is that companies pour in huge amounts of money and research in return for the right to reap profits from their drugs and vaccines. Thus, in their view, freely sharing their intellectual property, even during a global pandemic, is an unfair precedent to set.
But others have disagreed: "Manufacturers have received between $2 billion and $4 billion of public funds for research and development, including advance purchase agreements," Bozorgmehr said. The "cost for research and development should therefore be more than covered," he added.
Many have pointed to the fact that COVID-19 vaccines could generate billions of dollars in revenue for the companies.
For example, the Moderna vaccine, which was co-developed with the U.S. government and supported with $483 million in taxpayer backing, is expected to bring in $18.5 billion for the company this year, the company said in February.
But pharmaceutical companies do not seem to be budging on allowing free licensing, saying that instead of lifting IP restrictions, rich countries should simply give more vaccines to poorer countries through COVAX, the public-private initiative WHO helped create for more equitable vaccine distribution.
The organization and its partners delivered its first doses in very limited quantities last week, but many, including the WHO, are still concerned that rich countries are still not doing enough to share what they have, and when they do, it is mainly to drive diplomacy – pointing for example to the EU's export controls on vaccines.

